COVID-19 VACCINATION INFORMATION FOR RESIDENTS, FAMILIES AND FRIENDS
From Dr. Dueck, the Medical Director at Menno Place
We have been advised that Menno Place has been selected as one of the first Long Term Care sites to have Residents receive the Pfizer Covid 19 Vaccination.
This is VERY exciting news since it means that we have the potential to relax restrictions and enjoy closer family contact already by early in the New Year. This has been a LONG time coming and we are thrilled to be able to advise you of this development.
There is a challenge, however, that goes along with this news. We MUST have everything in place by the end of the day on Monday December 28 for this to happen.
What are we doing to get ready?
- Physicians and Pharmacists reviewing EVERY resident’s records to ensure we have identified anyone with a contraindication (a reason NOT to give this vaccination).
- Based on the above review process, an Order by a physician will be given to administer the Covid 19 Vaccination PROVIDED consent by the resident (if capable) or family is given.
- Asking all substitute decision makers to provide consent for their lovedone to have the vaccination administered (use the form on this page).
- Speaking with you should you wish to have individual conversation before giving consent. We are committed to having those conversations and will be calling all medical decision makers on Sunday, Dec. 27th and Monday, Dec. 28th.
- Organizing the teams of people who will bring the vaccine and administer it on Tuesday, December 29th.
I am grateful for the gift that this news brings at this special time of year. I am excited to look forward to seeing many of you in person again in just a few months’ time as we begin to regain the opportunity to be physically present with these dear people who have been isolated for far too long.
Best wishes,
Ken Dueck. Medical Director for Menno Place
Special instances where a physician will provide advice about receiving the vaccine:
1. Allergy to Polyethylene Glycol (PEG)
An allergy to Polyethylene Glycol (PEG) is the only significant concern for allergy with this particular vaccine.
-
- PEG is something commonly used in treatments for constipation (eg. RestoraLAX) with only RARE allergies occurring in the general population.
- MOST Long term care residents will have received PEG at some point to treat constipation as this is a very common problem in the frail elderly and PEG is a common treatment. In over 30 years of Medical Practice, I (Dr. Ken Dueck) have never personally seen anyone with an allergy to PEG so, this is extremely unlikely.
- If someone has a History of this rare allergy or has a history of Anaphylaxis (severe allergic reaction requiring the administration of adrenaline) in the past, they should NOT receive the vaccination at this time.
2. History of Anaphylaxis requiring administration of adrenaline
If someone has a History of this rare allergy (PEG) or has a history of Anaphylaxis (severe allergic reaction requiring the administration of adrenaline) in the past, they should NOT receive the vaccination at this time.
3. Immunocompromised - click to read the details
While being “immunocompromised” (eg chemotherapy, immunosuppression meds for diseases like Rheumatoid arthritis, blood diseases that reduce white cell counts etc.) reduce the likelihood of the vaccination being effective, it does NOT provide a safety concern and these individuals can still receive the vaccine. They can STILL get the Vaccine.
4. Recently vaccinated
Any other vaccination within the past 14 days is a reason NOT to give this vaccine at the present time but rather to wait until at least 14 days has passed.
5. Currently seriously ill
If someone is seriously ill at present (either with Covid 19 itself OR with any other serious illness such as pneumonia).
6. People who have already HAD COVID-19 (read the details)
Also, people who have already HAD Covid 19 and have recovered are still advised to receive the Vaccine since the immunity from previous infection is not as reliable as the immunity from getting the vaccine.
COVID-19 VACCINATION FAQs
COVID-19 mRNA Vaccine (Pfizer-BioNTech) FAQs
Facts about COVID-19 Vaccines
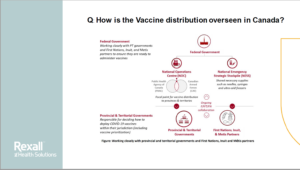
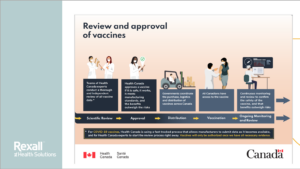
COVID Vaccine - How is it approved and administered in Canada?
This presentation outlines how Canadian vaccines are approved and Frequently Asked Questions about the ones approved in Canada – CLICK HERE
Click here to add your own text